Viroids were originally thought to be either the evolutionary precursors or degenerate decendents of conventional viruses. However, viroids' lack of sequence or structural similarity and lack of coding RNA ruled out this theory. Viroids are now commonly thought of as remnants from the "RNA world" since they are composed of only RNA and have ribozyme activity (Diener 2006).
Some have proposed that viroids are evolutionarily related to transposable elements or retroviral proviruses based on a common conserved central region and other characteristics such as the presence of specific repeat sequences (Kiefer et al 1983). Viroids also have some similarities to specific mitochondrial plasmids of Neurospora crassa and N. intermedia, two types of bread mold. The mitochondrial Varkud Small Plasmid (VSP) of N. intermedia exists mostly as a single-stranded RNA, and its linear form is generated in a process similar to that of viroids. Finally, sequences similar to the conserved elements of self-splicing group 1 introns (found in bacteria, lower eukaryotes, and higher plants) are present in most viroids (Diener 1996).
Sequencing of 15 viroids suggests a monophyletic origin of the RNAs. Sequence similarity between different viroids suggests that intermolecular recombinations and intramolecular rearrangements have occured during viroid evolution. The Columnea latent viroid CVLd is likely a mosaic of sequences from an HSVd-like viroid and one or more PSTVd-like viroids. Viroids are also similar to other plant RNA satellite viruses and the hepatitis delta satellite virus (Diener 1996).
1. ARS Timeline- Viroid Discovery <http://www.ars.usda.gov/IS/timeline/viroid.htm>
2. Wikipedia Viroid entry <http://en.wikipedia.org/wiki/Viroid>
RNA polymerase II and NEP (or PEP) are host DNA-dependent RNA polymerases necessary for transcription of Pospoviroidae and Avsunviroidae, respectively (Rackwitz et al 1981; Navarro et al 2000; Motard et al 2008). Please refer to biogenesis for more information.
Components of the RNA silencing pathway are thought to be important mediators of viroid pathogenicity. These components include Dicer-like (DCL) and the RNA-dependent RNA polymerase RDR6 (Gómez et al 2009). See cellular functions for more information.
Hammerhead Ribozyme Activity of the Family Avsunviroidae:
Viroids of the family Avsunviroidae possess a hammerhead ribozyme structure that auto-catalyzes cleavage of the oligomeric forms of RNA to the monomeric forms. In the presence of a divalent metal ion, the hammerhead ribozyme self-cleaves at a phosphodiester bond via a phosphodiester isomerization reaction. The reaction produces a 5' product with a 2',3' cyclic phosphodiester terminus and a 3' product with a 5' hydroxyl terminus (Flores 2004).
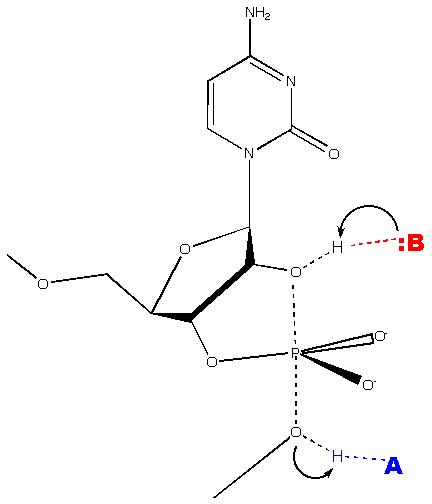
(Wgscot, Creative Commons License 2.5)
The hammerhead structure consists of a central catalytic core of conserved nucleotides flanked by three double-helices (stems I-III) with variable sequences.
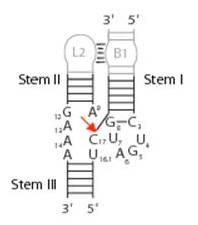
(Wgscott, Creative Commons License 2.5)
Ribozyme activity of Avsunviroidae is tightly regulated during viroid replication to activate cleavage of oligomeric RNAs and suppress cleavage of monomeric RNAs. The hammerhead structures of some viroids, such as the ASBVd RNAs, are thermodynamically unstable because their stem III consists of only two base pairs and a short 2-3 residue loop. This makes cleavage of these monomeric forms very thermodynamically unfavored. However, in the oligomeric state, two single hammerhead structures can pair to form a more stable double-hammerhead structure that promotes efficient cleavage of this oligomeric form. In other viroids such as PLMVd, CChMVd, and ELVd, the formation of monomeric hammerhead structures is not favored due to the presence of alternative thermodynamically stable structures. Therefore, the catalytically active hammerhead structures form only transiently during transcription, preferentially cleaving oligomeric RNAs (Flores 2004).
Viroids are plant pathogens that replicate in the nucleus (Pospoviroidae) or chloroplast (Avsunviroidae) of plants. They traffic from cell to cell via plasmodesmata. This trafficking is mediated by specific sequences or structural motifs withing the viroid (Ding et al 1997). Nuclear import also seem to be mediated by specific sequences within the viroid (Martinez de Alba et al 2003). The signals mediating import into the chloroplast remain unknown. Viroids use the plant phloem and vascular structure for long-distance trafficking (Tsagris et al 2008). Pospoviroidae form a complex with the RNA-binding phloem protein PP2, which increased systemic trafficking and translocation through grafts (Daròs et al 2006).
Infected plants can be asymptomatic or may display a variety of symptoms ranging from mild growth retardation to severe deformation, necrosis, chlorosis, or stunting. Symptoms depend on the strain of infected plant and environmental conditions (Tsagris et al 2008).
It is thought that the pathogenicity of viroids is due to viroid-specific siRNA silencing. Viroids are targets and inducers of the plant RNA silencing machinery. Small 21-25 nucleotide RNAs are observed in tissues from infected plants. These siRNAs are produced by the activity Dicer-like (DCL) on double-stranded RNA's. Viroid-specific siRNAs likely originate from the genomic viroid RNA present in the cytoplasm, which is used as a template for RNA-dependent RNA polymerases such as RDR6 to generate dsRNA that is then cleaved by DCL to produce siRNAs.
Although there is not a one-to-one correlation between siRNA production and pathogenicity, there is much evidence supporting the RNA-silencing induced pathogenicity model. Transgenic tomato plants expressing a truncated PSTVd incapable of infection causes the production of PSTVd-specific siRNAs and disease symptoms similar to that of full length PSTVd. In addition, disease symptoms of plants infected by HSVd do not depend on viroid accumulation but are instead dependent on RDR6 activity (Gómez et al 2009).
1. Diener TO (2003) Discovering viroids -- a personal perspective. Nature Reviews Microbiology 1, 75-80. PMID 15040183
2. Tsagris EM et al (2008) Viroids. Cellular Microbiology 10, 2168-2179. PMID 18764915
3. Gómez G et al (2009) Interplay Between Viroid-Induced Pathogenesis and RNA Silencing Pathways. Trends Plant Science [Epub ahead of print]. PMID 19375972
1. Diener TO (2003) Discovering viroids -- a personal perspective. Nature Reviews Microbiology 1, 75-80. PMID 15040183
2. Flores R et al (1998) A proposed scheme for viroid classification and nomenclature. Arch Virol 143, 623-629. PMID 9572562
3. Diener TO (1971) Potato Spindle Tuber "Virus" IV. Virology 45, 411-428. PMID 5095900
4. Diener TO (1996) Origin and Evolution of Viroids and Viroid-like Satellite RNAs. Virus Genes 11, 119-131. PMID 8828140
5. Kiefer MC et al (1983) Strutural Similarities Between Viroids and Transposable Genetic Elements. PNAS 80, 6234-6238. PMID 6312450
6. Daròs J et al (2006) Viroids: an Ariadne's Thread into the RNA Labyrinth. EMBO Reports 7, 593-598. PMID 16741503
7. Tsagris EM et al (2008) Viroids. Cellular Microbiology 10, 2168-2179. PMID 18764915
8. Rackwitz HR et al (1981) DNA-dependent RNA polymerase II of Plant Origin Transcribed Viroid RNA into Full-Length Copies. Nature 291, 297-301. PMID 7231549
9. Navarro JA et al (2000) A Chloroplastic RNA Polymerase Resistant to Tagetitoxin is Involved in Replication of Avocado Sunblotch Viroid. Virology 268, 218-225. PMID 10683343
10. Motard J et al (2008) The Peach Latent Modaic viroid Replication Initiation Site is Located at a Universal Position that Appears to be Defined by a Conserved Sequence. Virology 373, 362-375. PMID 18190946
11. Ding B et al (1997) Cell-to-cell Movement of Potato Spindle Tuber Viroid. Plant J 12, 931-936. PMID 9375403
12. Martinez de Alba et al (2003) A Bromodomain-Containing Proein from Tomato Specifically Binds Potato Spindle Tuber Viroid RNA in Vitro and in Vivo. J Virology 77, 9685-9694. PMID
13. Gómez G et al (2009) Interplay Between Viroid-Induced Pathogenesis and RNA Silencing Pathways. Trends Plant Science [Epub ahead of print]. PMID 19375972
14. Flores R et al (2004) Viroids: the Minimal Non-coding RNAs with Autonomous Replication. FEBS Letters 567, 42-48. PMID 15165891
15. Citti L and Rainaldi G (2005) Synthetic Hammerhead Ribozymes as Therapeutic Tools to Control Disease Genes. Curr Gene Ther 5, 11-24. PMID 15638708
16. Lewin AS and Hauswirth WW (2001) Ribozyme Gene Therapy: Applications for Molecular Medicine. Trends in Molecular Medicine 7, 221-228. PMID 11325634
Viroids replicate by RNA-RNA transcription, via rolling circle replication. In the Pospiviroidae (PSTVd-like) family, the circular plus strand RNA is transcribed by a host RNA polymerase into oligomeric minus strands and then oligomeric plus strands. These oligomeric plus strands are cleaved by a host RNase and ligated by a host RNA ligase to reform the monomeric plus strand circular RNA. This is called the asymmetric pathway of rolling circle replication (Daròs et al 2006).
The Avsunviroidae family (ASBVd-like) of viroids replicates via a symmetric pathway of rolling circle replication. In this mode of replication, oligomeric minus strands are first cleaved and ligated to form monomeric minus strands, which are then transcribed into oligomeric plus strands. These oligomeric plus strands are cleaved and ligated to reform the monomeric plus strand. Cleavage of the oligomeric plus and minus strands is mediated by the self-cleaving hammerhead ribozyme structure present in the Avsunviroidae family (and absent in the Pospiviroidae family) (Tsagris et al 2008).
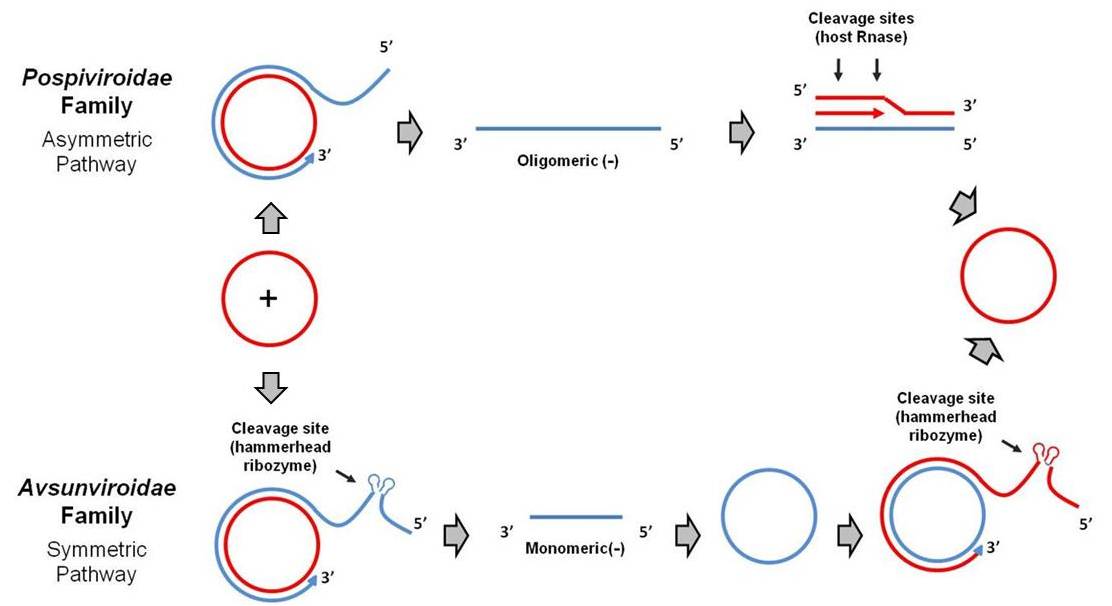
Viroids of the Pospiviroidae family are localized to the nucleus, while viroids of the Avsunviroidae family localize to chloroplasts. The nuclear viroids are dependent upon host DNA-dependent RNA-polymerase II for replication (Rackwitz et al 1981), and the chloroplastic viroids are dependent on either nuclear-encoded but chloroplast-localized DNA-dependent RNA-polymerase (NEP) (Navarro et al 2000) or plastid-encoded DNA-depedent RNA polymerase (PEP) (Motard et al 2008).
The potato spindle tuber viroid (PSTVd, Pospiviroids genus) was the first discovered viroid (Diener 2003). This viroid causes potatoes to be spindly, deformed, and often small. Infected and normal potatoes are show in the image below.
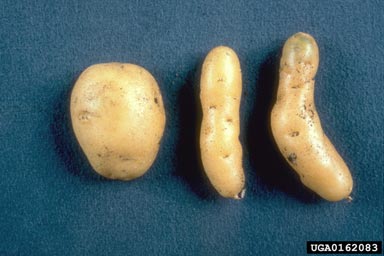
(Plant Protection Service Archive, Plant Protection Service, Bugwood.org)
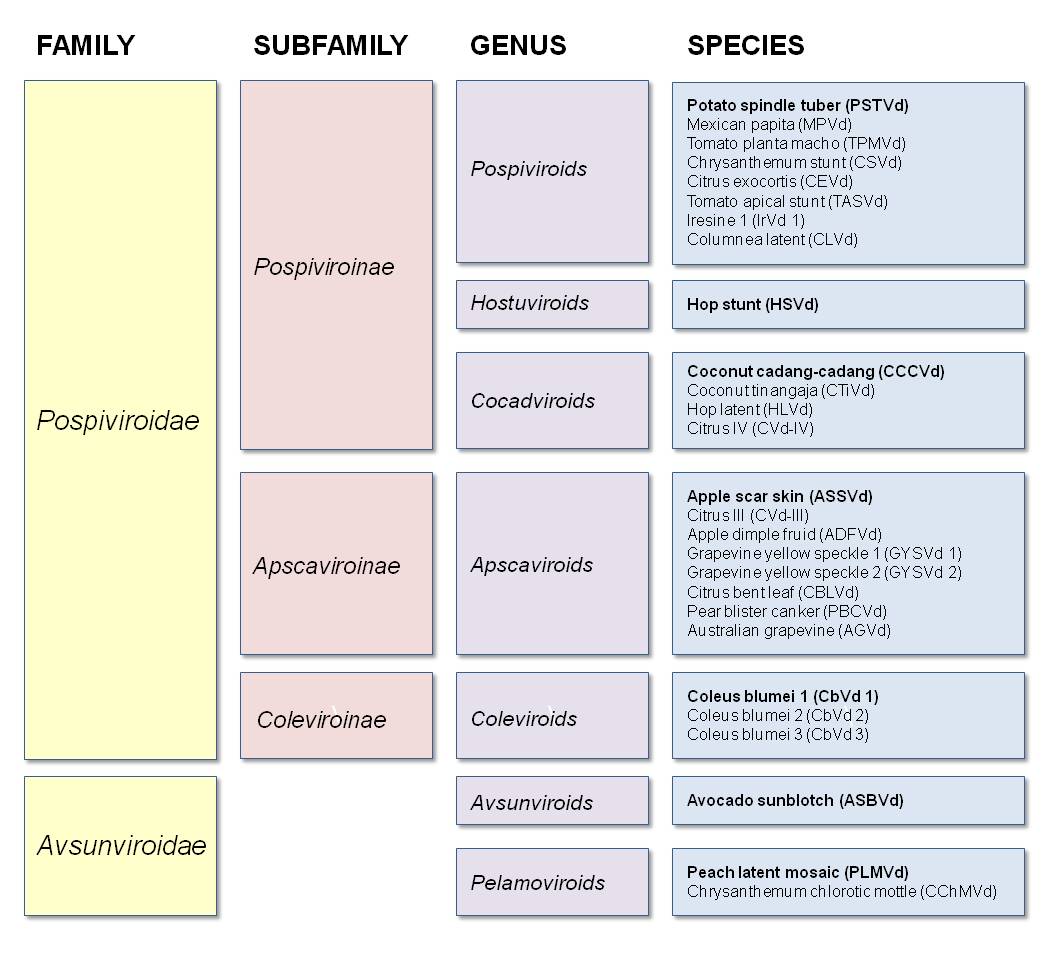
Other Members (Flores et al 1998):
Genus Pospiviroids:
- PSTVd (potato spindle tuber)
- MPVd (Mexican papita)
- TPMVd (tomato planta macho)
- CSVd (chrysanthemum stunt)
- CEVd (citrus exocortis)
- TASVd (tomato apical stunt)
- IrVd 1 (iresine 1)
- CLVd (columnea latent)
Genus Hostuviroids:
- HSVd (hop stunt)
Genus Cocadviroids:
- CCCVd (coconut cadang-cadang)
- CTiVd (coconut tinangaja)
- HLVd (hop latent)
- CVd-IV (citrus IV)
Genus Apscaviroids:
- ASSVd (apple scar skin)
- CVd-III (citrus III)
- ADFVd (apple dimple fruit)
- GYSVd 1 (grapevine yellow speckle 1)
- GYSVd 2 (grapevine yellow speckle 2)
- CBLVd (citrus bent leaf)
- PBCVd (pear blister canker)
- AGVd (Australian grapevine)
Genus Coleviroids:
- CbVd 1 (coleus blumei 1)
- CbVd 2 (coleus blumei 2)
- CbVd 3 (coleus blumei 3)
Genus Avsunviroids:
- ASBVd (avocado sunblotch)
Genus Pelamoviroids:
- PLMVd (peach latent mosaic)
- CChMVd (chrysanthemum chlorotic mottle)
Synthetic viroid-like hammerhead ribozymes as therapeutic tool:
Synthetic viroid-like ribozymes have been designed that act in trans to cleave RNA sequences of interest. These synthetic ribozymes consist of a target-binding domain, a catalytic domain, and a structural domain. The two arms of the target-binding domain are designed to base pair with the target RNA to form stems I and III of the typical hammerhead structure (see mechanism of action for figure). This base pairing confers target specificity. The catalytic domain is analogous to the conserved core of hammerhead ribozymes and connects stem I to stem II and stem II to stem III. The structural domain forms stem II, which is necessary to maintain the correct conformation of the catalytic domain (Citti and Rainaldi 2005).
Ribozymes can be delivered directly after modifying the 3' end and the 2' positions of pyrimidines to stabilize the molecule. Ribozyme-liposome conjugates are concentrated in the liver and lung after intravenous injection. An alternative is use of retroviral vectors to deliver genes encoding ribozymes. Engineered ribozymes have been explored as potential anti-cancer and anti-viral therapies. They also have the potential in treating dominant genetic disorders (Lewin and Hauswirth 2001).
The term viroid (meaning "virus-like") was coined by Theodor Diener, the discoverer of these small circular plant pathogens (Diener 2003). Viroids are divided into two families named for the prototypical member of each family: Pospiviroidae (from potato spindle tuber viroid, PSTVd) and Avsunviroidae (from avocado sunblotch viroid, ASBVd).
Pospiviroidae is further subdivided into three subfamilies: Pospiviroinae (from PSTVd), Apscaviroinae (from apple scar skin viroid, ASSVd), and Coleviroinae (from coleus blumei viroid 1, CbVd1). The subfamily Pospiviroinae contains three genera: Pospiviroids (from PSTVd), Hostuviroids (from hop stunt viroid, HSVd), and Cocadviroids (from coconut cadang-cadang viroid, CCCVd). The Apscaviroinae and Coleviroinae subfamilies each contain one genus, Apscaviroids and Coleviroids, respectively.
The Avsunviroidae family contains two genera: Avsunviroids (from ASBVd) and Pelamovirods (from peach latent mosaic viroid, PLMVd) (Flores et al 1998).
Viroids were identified as part of the effort to isolate the putative virus that caused potato spindle tuber disease. This disease leads to a second-year harvest of spindly, deformed potatoes. In the early 1960s, William B. Raymer, a plant pathologist at the US Department of Agriculture and Agricultural Research Service (ARS), initiated the project that would lead to the discovery of the viroid. At the time, he was working in the ARS Potato Diseases Investigation Laboratory in Beltsville, Maryland. Raymer and another ARS pathologist, Muriel O'Brien, came up with the first bioassay for the infectious agent that caused potato spindle tuber disease. They were able to transmit the pathogen to tomatoes, which exhibited stunted growth within two weeks of infection (ARS Timeline). With this ready source of diseased leaves, Raymer and O'Brien sought to isolate the virus using standard centrifugation techniques. However, Raymer found that he could not isolate the infectious fraction even at centrifugal forces high enough to sediment all previously identified viruses (Diener 2003).
In 1965, Raymer went to Theodor O. Diener, a member of the new ARS Plant Virology Pioneering Laboratory (ARS Timeline). Using the newly developed density gradient centrifugation technique, they confirmed that the pathogen was not a typical viral nucleoprotein particle. In a series of experiments, they next demonstrated that the infectious agent was free RNA. Extracts from infected plants remained pathogenic when treated with deoxyribonuclease or proteases, but lost infectiousness when treated with ribonuclease (Diener 2003). In 1966, Raymer left the project to take a job in industry. Diener went on to show by density-gradient centrifugation and gel electrophoresis that the infectious RNA was too small to contain the genetic information necessary for an autonomously replicating virus, and he coined the name "viroid" in 1971 to describe this new class of pathogen (Diener 1971).
Other researchers had also identified plant pathogens with properties not consistent with the conventional virion model. In 1968, RH Lawson published a report that the pathogen in another plant disease, chrysanthemum stunt, had properties different from those of a conventional virus. In 1968 and 1972, Semancik and Sanger published similar results about another presumed viral disease, citrus exocortis. It was soon found that these pathogens were also viroids, named chrysanthemum stunt viroid (CSVd) and citrus exocortis viroid (CEVd). By 1973, with the identification of three independent diseases caused by viroids, the concept of the viroid was firmly established (Diener 2003).
Further work (Diener 2003):
1973: First electron micrograph of a viroid shows its hairpin structure
1974: Confirmation that the viroid RNA does not encode any protein
1976: Electron microscopy demonstrates that viroids form closed circular RNAs
1978: The potato spindle tuber viroid (PSTVd) becomes the first eukaryotic pathogen to be sequenced

