Y RNAs have been found in all vertebrates in which they have been studied, as well as in C. elegans and the bacterium D. radiodurans. There are usually 1-4 Y RNAs present in a given species, and these Y RNA genes are usually found within a cluster on the same chromosome. They contain their own promoters and the distance between the genes as well as their order is well-conserved (2).
Studies surrounding the evolution of Y RNA genes indicate that a single ancestral Y RNA duplicated to give rise to two distinct Y RNA genes, which further gave rise to four Y RNA genes in the order Y5-Y4-Y3-Y1. The presence of less than 4 Y RNAs in some species suggests that some of these genes were lost through evolution (2).
Northern blot analysis of various vertebrate Y RNA samples probed with human Y cDNA showed the Y3 is the most conserved, Y1 is less conserved than Y3, and Y4 and Y5 are the least conserved (3).
The RoRNP consists of two proteins that interact directly with Y RNA, Ro60 and La. La contains an amino terminal RNA binding region (RNP-80) and carboxy terminus phosphorylation sites that modulate RNA binding ability. La binds to an oligouridine stretch found at the 3' end of Y RNA. Ro60 also contains an amino terminal RNA binding domain (RNP-80), and its overall conformation is also important for proper binding to Y RNA. Ro60 binds to the distal stem region of Y RNA at conserved nucleotides found within a bulged structure. Interaction of La and Ro60 with Y RNA have been proposed to protect and stabilize Y RNA, as well as aid in its translocation from the nucleus to the cytoplasm (10), (12). As a general mechanism, Y RNA binding to La and Ro may inhibit the chaperone activity of these proteins (9), (11).
hY1 and hY3 have also been shown to interact more transiently with hnRNP K and I and nucleolin in the loop region. The significance of this is not as well studied, but may involve regulation of the chaperone activities of these RNPs and proteins (9).
Y RNA functionally interacts with components of the DNA replication machinery, such as RPA and PCNA (15).
Studies revealing the structure of Ro60 suggest that Y RNA has the ability to inhibit Ro60's chaperone activity by binding to it (11). Ro60 binds misfolded RNAs via the external region of its N-terminal circular domain. 3' single stranded regions of bound RNA are threaded through the central cavity of this circle, and this is thought to be crucial for targeting misfolded RNAs for degradation or possibly for facilitating refolding. Y RNAs bind Ro60 at this same external region of the circular domain via their stem region. This sterically hinders the ability of misfolded RNAs to bind Ro60, implicating Y RNAs as a repressor of Ro60 activity. Similarly, regions in Ro60 required for its translocation to the nucleus seem to be masked by binding of Y RNA, and binding of Y RNA to hnRNP K and I has been shown to inhibit their splicing related chaperone activities (9), (12),
The mechanism for RoRNP mediated resistance to UV irradition in D. radiodurans and mouse ES cells is unknown (7), (14).
The mechanism for Y RNA's role in DNA replication is unknown. It does not appear that Y RNA functions as a primer, and it does not involve interaction of Y RNA with Ro60 (15).
Y RNAs are part of the RoRNP, which also contains the proteins Ro60 and La. Ro60 acts as a molecular chaperone that regulates proper folding and assembly of small, non-coding RNAs by binding them. For example, roles for Ro60 in regulating the maturation of U2 snRNAs, 23S rRNAs, and 5s rRNAs has been observed in mouse ES cells, D. radiodurans, and X. laevis, respectively (6), (7), (8). La also appears to have chaperone activities involved in regulating processing of RNAs and assembly of RNPs (9), (10). Binding of Y RNA to Ro60 and La is thought to repress these chaperone activities (9), (11). Similarly, hY1 and hY3 may inhibit chaperone activities of the more transient interacting partners, heterogeneous nuclear RNP (hnRNP) K and I (9). The most recent reports also suggest Y RNA regulates the localization of Ro60 (12). While the functional roles of the different Y RNAs have not been elucidated, it has been proposed that their structural differences may confer the ability of the RoRNP to recognize different types of misfolded RNAs (13).
Studies in mammalian cells and D. radiodurans suggest that the Ro60 and Y RNA may also be important in resistance to UV irradiation. Increases in RoRNP particles (i.e. increases in levels of Ro and binding of Y RNA to Ro) in D. radiodurans and increases in the nuclear localization of RoRNPs in mouse embryonic stem cells occurs in response to UV irradiation, and inhibition either of these processes decreased survival, implicating a role for RoRNPs and Y RNAs in UV resistance, however the mechanism behind this is not known (7), (14).
Y RNA has also been shown to have an essential role in DNA replication. Studies using isolated human nuclei in cell free DNA replication systems were used to show that hY1, hY3, hY4, or hY5 is required but not sufficient for replication in this system. This data also showed that there is redundancy among the hY RNAs in regulating DNA replication. These results were supported by in vitro data showing that siRNA knockdown of hY1 in HeLa cells decreased proliferation (15).
Wolin, S.L. and Reinisch, K.M. (2005) The Ro 60 kDa autoantigen comes into focus: Interpreting epitope mapping experiments on the basis of structure. Autoimmun Rev 5:367-372. PMID: 16890888
Reinisch, K.M. and Wolin, S.L. (2007) Emerging themes in non-coding RNA quality control. Curr Opin Struct Biol 17:209-214. PMID: 17395456
Hogg, J.R. and Collins, K. (2008) Structured non-coding RNAs and the RNP Renaissance. Curr Opin Chem Biol 12:684-689. PMID: 18950732
(1) Lerner, M.R. et al. (1981) Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science 211:400-402. PMID: 6164096
(2) Perreault, J., Perreault, J.P., and Boir, G. (2007) Ro-Associated Y RNAs in Metazoans: Evolution and Diversification. Mol Biol Evol 24:1678-1689. PMID: 17470436
(3) Farris, A.D., O'Brien, C.A., and Harley, J.B. (1995) Y3 is the most conserved small RNA component of the Ro ribonucleoprotein complexes in vertebrate species. Gene 154:193-8. PMID: 7534247
(4) Hendrick, J.P. et al. (1981) Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol Cell Biol 1:1138-49. PMID: 6180298
(5) Teunissen, S.W. et al. (2000) Conserved features of Y RNAs: a comparison of experimentally derived secondary structures. Nucleic Acids Res 28:610-9. PMID: 10606662
(6) Chen, X. et al. (2007) An ortholog of the Ro autoantigen functions in 23S rRNA maturation in D. radiodurans. Genes Dev 21:1328-1339. PMID: 17510283
(7) Chen, X. et al. (2003) The Ro autoantigen binds misfolded U2 small nuclear RNAs and assists mammalian cell survival after UV irradiation. Curr Biol 13:2206-11. PMID: 14680639
(8) O'Brien, C.A. and Wolin, S.L. (1994) A possible role for the 60 kD Ro autoantigen in a discard pathway for defective 5S ribosomal RNA precursors. Genes Dev 8:2891-2903. PMID: 7995526
(9) Belisova, A. et al. (2005) RNA chaperone activity of protein components of human Ro RNPs. RNA 11:1084-1094. PMID: 15928345
(10) van Venrooij, W. J., Slobbe, R.L., and Pruijn, G. J.M. (1993) Structure and function of La and Ro RNPs. Mol Biol Reports 18:113-119. PMID: 7694079
(11) Stein, A.J. et al. (2005) Structural Insights into RNA Quality Control: The Ro Autoantigen Binds Misfolded RNAs via Its Central Cavity. Cell 121:529-539. PMID: 15907467
(12) Sim, S. et al. (2009) The subcellular distribution of an RNA quality control protein, the Ro autoantigen, is regulated by noncoding Y RNA binding. Mol Cell Biol 20:1555-64. PMID: 19116308
(13) Hogg, J.R. and Collins, K. (2008) Structured non-coding RNAs and the RNP Renaissance. Curr Opin Chem Biol 12:684-689. PMID: 18950732
(14) Chen, X., Quinn, A.M., and Wolin, S.L. (2000) Ro ribonucleoproteins contribute to the resistance of Deinococcus radiodurans to ultraviolet irradiation. Genes Dev 14:777-82. PMID: 10766734
(15) Chistov, C.P. et al. (2006) Functional requirement of noncoding Y RNAs for human chromosomal DNA replication. Mol Cell Biol 26:6993-7004. PMID: 16943439
(16) Christov, C.P., Trivier, E., and Krude, T. (2008) Noncoding human Y RNAs are overexpressed in tumors and required for cell proliferation. Br J Cancer 98:981-8. PMID: 18283318
Y RNAs are transcribed by RNA polymerase III (RNAPIII). They generally contain 85-112 nucleotides. Both mouse and human forms of Y RNA have been found to contain few, if any modified nucleotides. mY1 and mY2 are pyrimidine rich, and similarly, hY4 and hY5 contain a high proportion of uracil nucleotides (4).
Structural analysis indicated that the mature form of Y RNA contains a loop structure and a double stranded stem region formed by pairing of the 5' and 3' ends. The stem also contains a bulged helix region within the stem that is critical for binding to Ro60 during formation of the RoRNP. They also contain a single stranded 3'-poly(U) tail that is important for binding to La, another component of the RoRNP (5).
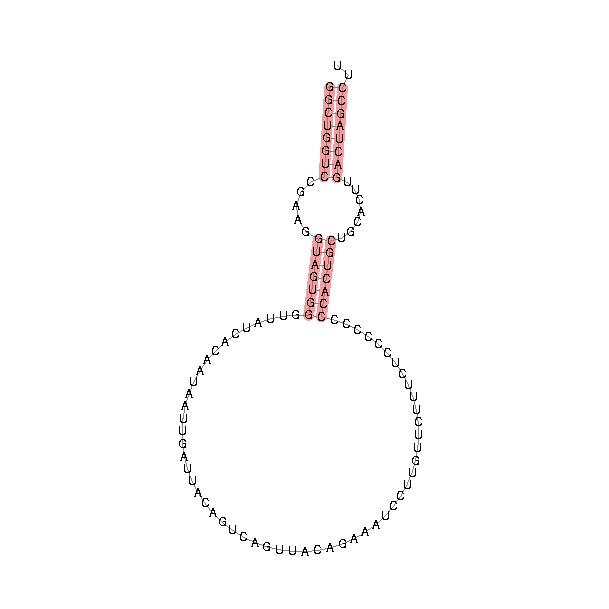
General structure of a Y RNA. Note the large loop structure, the double stranded stem region that contains a bulged area that is critical for Ro60 binding, and the 3'-poly(U) region critical for La binding. Different Y RNAs contain slight variations of the loop and stem regions.
(Image from wikipedia: The right to use this work is granted to anyone for any purpose, without any conditions, unless such conditions are required by law.)
Human: hY1, hY3, hY4, hY5
Mouse: mY1 and mY3
C. elegans: CeY
X. laevis: xYa, xY5
Human Y RNA was shown to be upregulated in human cancer tissues and to be required for the increased proliferation of cancer cell lines. Based on this, it was suggested that Y RNAs could serve as a potential biomarker for identification of proper therapeutic intervention (16).
Also, when considering its role in cancer and its role in regulating DNA replication, this suggests that it could be used as a tool to manipulate cell cycle regulation and cell proliferation in culture. This would provide a way to study these processes by inducing them so that they could be regulated in an inhibitory manner.
These small, non-coding cytoplasmic localized RNAs were named with a Y prefix to distinguish them from nuclear small, non-coding RNAs (prefixed with a U) that are approximately the same size but were shown by two-dimensional oligonucleotide mapping to be a distinct species (1). Y RNAs from different species are generally distinguished by the inclusion of the first letter of the species preceding the Y. Different Y RNAs within a species are designated by numbers. (e.g. hY1 (human) or mY3 (mouse)).
Y RNAs were first identified during the characterization of serum from patients with the autoimmune disorder lupus erythematosus. Previously, autoantigens that antibodies from these patients reacted against were identified, but their molecular nature was unknown. Lerner et al. set out to determine this by characterizing the reactivity of serum from these patients with whole cell extracts from Ehrlich ascites cells. Analysis of ribonucleic acid content by gel electrophoresis and two-dimensional oligonucleotide mapping showed that small RNAs, which consisted of Y1, Y2, and Y3 RNAs were precipitated by serum containing antibodies against the proteins Ro and La, which are a components of the Ro ribonucleoprotein (RoRNP) suggesting that they bound these proteins and were consequently precipitated in a RNA-protein complex by these antibodies. Treatment of extracts such that protein was removed led to an inability of anti-Ro or -La antibody containing serum to precipitate Y RNAs, supporting this (1).
Because of their essential role in DNA replication, it was hypothesized that Y RNAs may have a critical role in maintaining the neoplastic phenotype of human tumors. Consequently, a study comparing the expression of Y RNA in tumor versus normal tissue demonstrated that human Y RNAs are upregulated in tumors. Accordingly, further analysis of the role of Y RNA in proliferation of human cancer cell lines indicated that the proliferative capacity of these cell lines is compromised by siRNA knockdown of Y RNA, suggesting Y RNA is required for tumorigenicity (16).

