Vaults have so far been found to be present in high numbers among higher eu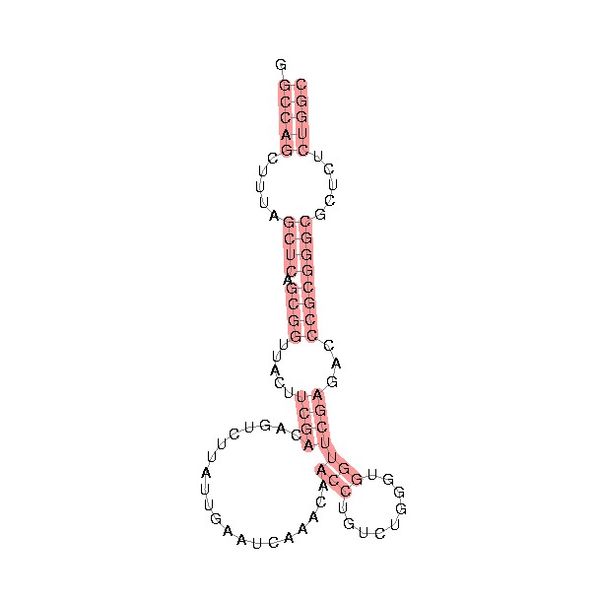 karyotes including mammals, amphibians, and avians, as well as lower eukaryotes including Dictyostelium discoideum. Both structure and protein composition are highly conserved among species suggesting that their function is crucial to eukaryotic cell function (3). vRNA has a species-specific length that ranges between 86 and 141 bases. Also, the number of vRNAs expressed differs from organism to organism. For example, rats and mice express a single vRNA 141 bases long while bullfrogs express 2 vRNAs: one 89 bases long and the other 94. Humans express three related vRNAs: hvg1 (98 bases), hvg2 (88 bases), and hvg3 (88 bases). It has been speculated that the function of the relatively long rodent vRNA is covered by multiple smaller vRNAs in other species (R1). Despite differences in vRNA between species, in all vRNAs the polymerase III promoter elements are highly conserved. In addition, all vRNAs are predicted to fold into similar stem-loop structures (4) (see figure to right. Figure is public domain, taken from the Rfam database).
karyotes including mammals, amphibians, and avians, as well as lower eukaryotes including Dictyostelium discoideum. Both structure and protein composition are highly conserved among species suggesting that their function is crucial to eukaryotic cell function (3). vRNA has a species-specific length that ranges between 86 and 141 bases. Also, the number of vRNAs expressed differs from organism to organism. For example, rats and mice express a single vRNA 141 bases long while bullfrogs express 2 vRNAs: one 89 bases long and the other 94. Humans express three related vRNAs: hvg1 (98 bases), hvg2 (88 bases), and hvg3 (88 bases). It has been speculated that the function of the relatively long rodent vRNA is covered by multiple smaller vRNAs in other species (R1). Despite differences in vRNA between species, in all vRNAs the polymerase III promoter elements are highly conserved. In addition, all vRNAs are predicted to fold into similar stem-loop structures (4) (see figure to right. Figure is public domain, taken from the Rfam database).
Vault RNA Wikipedia site - A very brief description of vRNAs
The Vault Website - Some very good information about vaults and vRNA, Hosted by the Rome lab at UCLA
vRNA associates with the vault complex. The Vault consists of multiple components. There is the MVP, which constitutes over 70% of the vault complex, and two minor proteins (1). One of the minor proteins (Mr 193,000) was found to contain poly(ADP-ribose) polymerase (PARP) activity and was named VPARP (16). The other minor protein (Mr 240,000), was identified as TEP1. TEP1 is shared with the telomerase complex (14).
A portion of the vRNA associates with the La autoantigen (17). The La autoantigen is an RNA-binding protein involved in initiation and termination of RNA polymerase III transcription (18). Additionally, the La autoantigen is a protein that co-purifies with vaults. The significance of this interaction is still not clear. It is possible that the La autoantigen acts as a chaperone (17).
Since the function of vRNA remains elusive, so does the mechanism of action. As mentioned above, it is hypothesized that at least in species with multiple vRNAs such as humans, the ratio of what vRNA species are associated with vaults may have a functional implication on drug resistance (10). vRNAs from different species are all predicted to form a stem-loop structure. The role of the stem-loop in vRNA is still unknown; however, it is possible that the loop regions may be involved in mechanism via interaction with other RNAs or proteins (4).Regulation of vRNA, as stated earlier is hypothesized to be controlled by the two closely spaced B boxes along with the 5’ flanking sequence (R3, 6).
As stated above, one of the minor vault proteins was found to be identical to mammalian TEP1. Using a yeast three-hybrid system, it was shown that murine TEP1 associates with human vRNAs (14). The vRNAs are found at the end of the vault caps, enabeling the vRNA to interact with both the interior and exterior regions of the vault (15). It is possible that the binding of vRNA with TEP1 determines whether TEP1 associates with vaults or with the telomerase complex (R1).
It is believed that the vRNA constitutes an essential functional role rather than a structural role. This conclusion was based on the observations that degradation of the vRNA had no effect on vault morphology and that the predicted secondary structures for vRNAs from different species are predicted to be highly conserved (5). Since their discovery in 1986, the function of vaults and vRNA remains speculative. Several groups have proposed that vaults play a role in intracellular transport based on their hollow barrel structure and their cellular localization (most of which reside in the cytoplasm) (R1, R3). Vaults also partially co-localize with the cytoskeleton suggesting that vaults may be involved in cytoskeleton maintanence or transported along the cytoskeleton. In the electric ray Torpedo, vaults were shown to be transported between soma and nerve terminals, supporting the role of vaults in cytoskeleton transport (7). It remains unknown if vaults and vRNAs are in fact involved in intracellular transport.
Lung resistance-related protein (LRP) is a multi drug resistance (MDR)-associated protein (MRP). MDR is a major cause of cancer treatment failure and has been found to be associated overexpression on MRPs. It was found that the cDNA coding for the LRP gene is the cDNA for the human major vault protein (8). It was found that MVP/vaults were overexpressed in many human tumor cell lines that had MDR phenotypes (9). It is possible that vaults act by transporting drugs away from their targets (R1).
With regard to the function of vRNA, it is notable that vRNAs are associated to the vault complex in several human cancer cell lines. The three human vRNAs are bound to the vault complex in ratios that do not reflect their expression levels. The three vRNAs have different affinities for telomerase-associated protein 1 (TEP1) (10). TEP1 was found to be identical to one of the minor vault proteins. Tep1 is an evolutionarily conserved component of the telomerase complex (11). Nearly all of the vRNA associated with vaults is hvg1; however, in at least a few of the MDR cell lines, hvg3 was found to be associated with the vaults at consistently higher levels. This observation suggests that the ratio of what vRNA species are associated with vaults may have a functional implication on drug resistance, and potentially cellular transport (10).
TEP1 KO mice have been generated. These mice contained vault particles but TEP1 and vRNA appeared to be lost, implicating that TEP1 is required for stable vRNA association to vaults. These mice, in addition to MVP knockout mice are viable, healthy, and display no obvious abnormalities (12, 13). The lack of phenotype in TEP1 KO mice suggests that vRNAs are either not as essential as their conservation predicts they are, or that they are functional in ways not involving interaction with vaults. It is also possible that the KO phenotype is subtle and more tests need to be performed in order to determine the function/importance of vaults and vRNA. MVP KO mice showed no change in sensitivity to drugs when compared to wild-type cells. These observations lead to the conclusion that at least in mice, vaults are not directly involved in drug resistance (R1).
R1. van Zon, A., et al., The vault complex. Cell Mol Life Sci, 2003. 60(9): p. 1828-37.
R2. Willis, I.M., RNA polymerase III. Genes, factors and transcriptional specificity. Eur J Biochem, 1993. 212(1): p. 1-11.
R3. Kickhoefer, V.A., S.K. Vasu, and L.H. Rome, Vaults are the answer, what is the question? Trends Cell Biol, 1996. 6(5): p. 174-8.
1. Kedersha, N.L. and L.H. Rome, Isolation and characterization of a novel ribonucleoprotein particle: large structures contain a single species of small RNA. J Cell Biol, 1986. 103(3): p. 699-709.
2. Nandy, C., et al., Epstein-Barr Virus-Induced Expression of a Novel Human Vault RNA. J Mol Biol, 2009. 17: p. 17.
3. Kedersha, N.L., et al., Vaults. II. Ribonucleoprotein structures are highly conserved among higher and lower eukaryotes. J Cell Biol, 1990. 110(4): p. 895-901.
4. Kickhoefer, V.A., et al., Vault ribonucleoprotein particles from rat and bullfrog contain a related small RNA that is transcribed by RNA polymerase III. J Biol Chem, 1993. 268(11): p. 7868-73.
5. Kedersha, N.L., et al., Vaults. III. Vault ribonucleoprotein particles open into flower-like structures with octagonal symmetry. J Cell Biol, 1991. 112(2): p. 225-35.
6. Vilalta, A., et al., The rat vault RNA gene contains a unique RNA polymerase III promoter composed of both external and internal elements that function synergistically. J Biol Chem, 1994. 269(47): p. 29752-9.
7. Herrmann, C., et al., The major vault protein (MVP100) is contained in cholinergic nerve terminals of electric ray electric organ. J Biol Chem, 1996. 271(23): p. 13908-15.
8. Scheffer, G.L., et al., The drug resistance-related protein LRP is the human major vault protein. Nat Med, 1995. 1(6): p. 578-82.
9. Siva, A.C., et al., Up-regulation of vaults may be necessary but not sufficient for multidrug resistance. Int J Cancer, 2001. 92(2): p.
10. van Zon, A., et al., Multiple human vault RNAs. Expression and association with the vault complex. J Biol Chem, 2001. 276(40): p. 37715-21.195-202.
11. Harrington, L., et al., Human telomerase contains evolutionarily conserved catalytic and structural subunits. Genes Dev, 1997. 11(23): p. 3109-15.
12. Kickhoefer, V.A., et al., The Telomerase/vault-associated protein TEP1 is required for vault RNA stability and its association with the vault particle. J Cell Biol, 2001. 152(1): p. 157-64.
13. Mossink, M.H., et al., Disruption of the murine major vault protein (MVP/LRP) gene does not induce hypersensitivity to cytostatics. Cancer Res, 2002. 62(24): p. 7298-304.
14. Kickhoefer, V.A., et al., Vaults and telomerase share a common subunit, TEP1. J Biol Chem, 1999. 274(46): p. 32712-7.
15. Kong, L.B., et al., RNA location and modeling of a WD40 repeat domain within the vault. Rna, 2000. 6(6): p. 890-900
16. Kickhoefer, V.A., et al., The 193-kD vault protein, VPARP, is a novel poly(ADP-ribose) polymerase. J Cell Biol, 1999. 146(5): p. 917-28.
17. Kickhoefer, V.A., et al., The La RNA-binding protein interacts with the vault RNA and is a vault-associated protein. J Biol Chem, 2002. 277(43): p. 41282-6.
18. Craig, A.W., et al., The La autoantigen contains a dimerization domain that is essential for enhancing translation. Mol Cell Biol, 1997. 17(1): p. 163-9.
19. Poderycki, M.J., et al., The vault exterior shell is a dynamic structure that allows incorporation of vault-associated proteins into its interior. Biochemistry, 2006. 45(39): p. 12184-93.
20. Raval-Fernandes, S., et al., Increased susceptibility of vault poly(ADP-ribose) polymerase-deficient mice to carcinogen-induced tumorigenesis. Cancer Res, 2005. 65(19): p. 8846-52.
As determined in HeLa S-100 in vitro transcription assays, the Rat vRNA is efficiently transcribed into a small, 141 base RNA product by RNA polymerase III. The RNA remains untranslated. The length of vRNA differs between species (see above). In all species examined so far, the vRNA constitutes less than 5% of the vault complex (1, 5). The vRNA contains two B-box elements and one A-box element (type-2 promoter elements). (4). A and B boxes are binding sites for TFIIIC which positions TFIIIB immediately upstream of the gene. Subsequently, TFIIIB directs binding of RNA polymerase III, which initiates transcription (R2). The vRNA contains a TATA box sequence at position -25 and an assumed proximal sequence element at position -70 (with respect to transcription initiation site). Additionally, viable 5’ flanking sequence is required for transcription. Also, at high transcription factor concentrations, the presence of the two B boxes inhibits vRNA transcription. It is postulated that the two closely spaced B boxes along with the 5’ flanking sequence provide a mechanism for the regulation of vRNA gene activity (R3, 6). The rat vRNA has a distinct tissue-specific pattern with levels in the spleen, intestine, heart and kidney 10 fold higher than in the brain (4).
The conserved hollow capsule shape of vaults along with their diverse localization have supported the notion that vaults are carriers or transporters in the cell. Additionally, vaults have been shown to be dynamic particles and that the protein shell formed by MVP can transiently open, allowing the incorporation of other vault components and perhaps vault-interacting proteins (19). One can imagine a situation where an investigator could take advantage of the hollow vault to transport drugs or other substances from one part of a cell to another. It is unclear if the vRNA would be necessary for this function. A clear use of the vRNA as a tool has yet to be found, other than to further study the effects of small RNAs on MDR. Along those lines, in the future, vaults may be used as a prognostic marker for MDR. The vRNA could be involved in prognosis based on the ratio of which vRNAs (hvg 1,2,3) interact with TEP1.
Vault RNA is also known as vRNA and vtRNA
In 1986, while purifying rat liver coated vesicles, the Leonard Rome lab of UCLA consistently observed ovoid structures that were smaller than most coated vesicles. After purification of these molecules, electron microscopy revealed highly uniform ovoid bodies measuring 35 x 65 nm possessing elaborate structure. The morphology of the purified structures somewhat resembled that of clathrin-coated vesicles; however, they contained no clathrin when examined by SDS PAGE. These particles were named vaults because of their resemblance to the multiple arches which form cathedral vaults. Vaults are large ribonucleoprotein particles found in eukaryotic cells composed of multiple copies of a Mr ~100,000 major vault protein (MVP) and two minor proteins of Mr ~193,000 and ~240,000. An additional major species was purified with an Mr of 37,000. This specie repeatedly remained unstained for the protein die Coomassie Brilliant Blue. Additionally, this vault specie was not degraded with treatment of proteinase K as did all other species. The mobility of this vault specie on SDS PAGE was unaffected by DNase I, but it was completely destroyed by treatment with RNase A. Other experiments showing that this specie stains with ethidium bromide and could be [32P]labeled using T4 ligase and T4 Kinase lead to the conclusion that a novel small RNA had been identified. This RNA was termed vault RNA (vRNA) and in rats was found be 141 bases. Further analysis of the RNA sequence confirmed the novelty of the vRNA (1).
There have been attempts to understand the role of vaults and perhaps a role in human disease by generating knockout models. In Dictyostelium, unlike in mammals, there are three major vault proteins. Disruption of two of these genes impedes growth, suggesting a role in cell proliferation and/or survival (R1). However, in mice (that contain one MVP like humans), disruption of MVP lead to a loss of vaults, but there was not observable phenotype. TEP1 KO mice (which are also devoid of vRNA) also have no observable phenotype (12). Even though cancer cell lines have shown over expression of MVP, MVP KO mice did not show an increased sensitivity to drugs compared with wild type (13). Interestingly, an increase in carcinogen-induced tumors in VPARP-deficient mice has been reported (20); however, vRNAs have yet to be linked to specific human diseases. Along with vRNA's known association with vaults and their potential cellular roles described above, it would not surprise me if vRNAs were subtly associated with numerous conditions.

